Every year, millions of people in New Zealand and around the world reach for vitamins and supplements like they’re just another painkiller or cold tablet. You grab that bottle from the pharmacy shelf, glance at the label, and assume it’s as safe and regulated as the ibuprofen next to it. But here’s the truth: OTC vitamins and supplements are not held to the same standards as OTC medications - and that gap could be putting your health at risk.
What’s on the label? Not nearly enough
If you’ve ever read the Drug Facts label on an OTC pain reliever, you know what to expect: active ingredients listed by exact milligram amounts, clear directions for use, warnings about who shouldn’t take it, possible drug interactions, and an expiration date. It’s standardized. It’s strict. It’s designed to keep you safe. Now look at the label on your multivitamin. It says "Supplement Facts." Sounds similar, right? But here’s where it falls apart. The Supplement Facts panel doesn’t require the same level of detail. You won’t find specific warnings about pregnancy risks, drug interactions, or how much sodium is in each serving - even if that sodium could raise your blood pressure. You won’t see clear dosage instructions for specific conditions. Instead, you get vague claims like "supports immune health" - with a tiny disclaimer that says, "This statement has not been evaluated by the FDA. This product is not intended to diagnose, treat, cure, or prevent any disease."The vitamin A trap
Let’s talk about vitamin A. If you’re pregnant or planning to be, you’ve probably heard you should avoid too much of it. Why? Because high doses of retinol - the active form of vitamin A - can cause serious birth defects. Prescription acne drugs like isotretinoin come with multiple warnings: pregnancy tests, birth control requirements, and bold labels. But a vitamin A supplement with 10,000 IU per capsule - the same amount that triggers those warnings in drugs - might only have a small, faint note buried in fine print. And even then, it might not even say "retinol." It might just say "vitamin A" in International Units (IU). That’s a problem. Because vitamin A can come from two sources: retinol (dangerous in excess) or beta-carotene (safe). The label doesn’t tell you which one you’re getting. So if you’re taking prenatal vitamins and think you’re safe because you’re "just taking a supplement," you might be unknowingly exceeding the safe limit. A 2021 study from the American College of Obstetricians and Gynecologists found that 40% of prenatal vitamins contain vitamin A levels above the recommended limit during pregnancy - and only 22% of them have clear, prominent warnings.Hidden ingredients and untested blends
Ever seen a supplement that lists a "proprietary blend"? That’s a red flag. It means the manufacturer isn’t required to tell you how much of each ingredient is actually in there. A 2022 analysis found that 63% of weight loss supplements and 41% of protein powders use these blends to hide potentially harmful doses. One product might say "1,000 mg blend" with five ingredients. But you have no idea if you’re getting 990 mg of filler and 10 mg of the active compound - or the reverse. And if one of those ingredients is a stimulant or a banned substance? You won’t know until you feel the side effects. The FDA doesn’t test supplements before they hit the shelves. They wait for reports of harm. Between 2008 and 2020, the FDA found 776 dietary supplements that contained undeclared pharmaceutical ingredients - like statins, erectile dysfunction drugs, or steroids - hidden in products sold as "natural" weight loss or muscle-building aids. And because these aren’t classified as drugs, they don’t need to prove safety before sale. They just need to be removed after someone gets hurt.
Why don’t they warn about drug interactions?
Think about this: You’re on blood thinners. You start taking a fish oil supplement because you heard it’s good for your heart. But fish oil can increase bleeding risk. Shouldn’t the label warn you? In OTC medications, yes - 100% of labels list drug interactions. In supplements? Only 17% do. A 2021 study in JAMA Internal Medicine confirmed it. That’s not a mistake. That’s the law. Pharmacists in Wellington and across New Zealand are seeing more patients come in confused. "Why does my painkiller say not to take it with aspirin, but my vitamin D doesn’t say anything about warfarin?" That’s a common question. Walgreens alone logged over 14,000 such inquiries in early 2023 - and the number keeps rising. People assume that if it’s sold next to medicine, it’s regulated like medicine. It’s not.Who’s really behind the label?
The supplement industry is big business. In 2022, it made $54.2 billion in the U.S. alone. That kind of money means lobbying power. The industry spent $8.2 million in federal lobbying in 2022 to block stricter labeling rules. Meanwhile, the FDA takes an average of 427 days to act on dangerous supplement reports - compared to 45 days for OTC drugs. That’s not inefficiency. That’s policy. Even when the FDA tries to improve things, progress is slow. In June 2023, they proposed new guidance for vitamin A labeling - suggesting manufacturers use mcg RAE (Retinol Activity Equivalents) instead of IU and add clear pregnancy warnings. But that’s still just a draft. It’s not law. And manufacturers don’t have to follow it.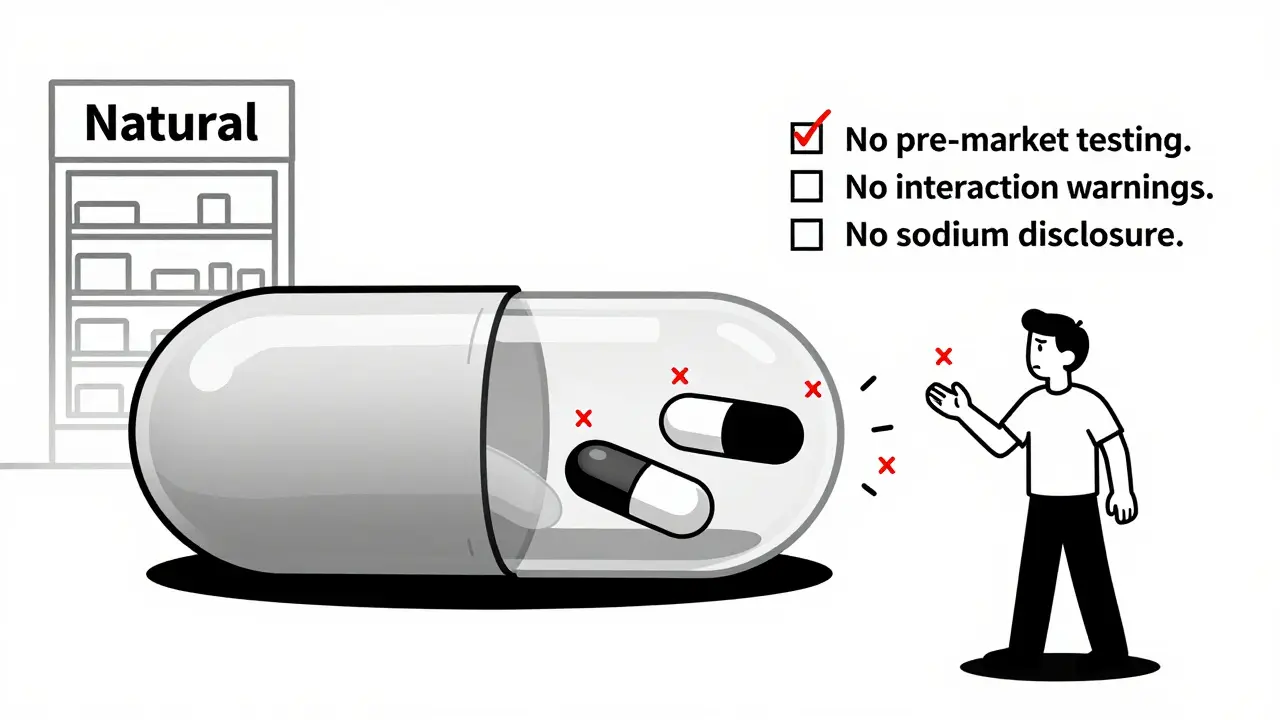
What you can do
Here’s the reality: You can’t rely on the label. But you don’t have to fly blind.- Look for third-party certifications: NSF, USP, or ConsumerLab. These organizations test for purity and label accuracy. There were 2,147 certified products in 2023 - up 37% from 2021.
- Use independent databases: The NIH’s Supplement Label Database now has over 65,000 products with standardized info. It’s free. It’s searchable. It’s not perfect, but it’s better than guessing.
- Check Examine.com: This nonprofit site breaks down supplement science with citations. Over 4.7 million people use it monthly. They don’t sell anything. They just tell you what the science says.
- Talk to your pharmacist or doctor before starting anything new - especially if you’re on medication, pregnant, or have liver or kidney issues.
- When in doubt, skip it. Vitamins aren’t magic. Most healthy people don’t need them. If you’re eating a balanced diet, you’re probably getting what you need.
The bigger picture
This isn’t about scaring you away from supplements. It’s about making sure you’re not fooled by the illusion of safety. The supplement industry thrives on the assumption that if it’s sold in a pharmacy, it’s been vetted. But it hasn’t. Not like medicine has. The Drug Facts label exists because people got hurt. The Supplement Facts label exists because companies fought to keep it simple. And right now, you’re the one paying the price - with your health.Do OTC supplements have to list drug interactions like OTC medications do?
No. OTC medications are required by law to list all known drug interactions. Dietary supplements are not. Only 17% of supplement labels include interaction warnings, compared to 100% of OTC drug labels. This is a major regulatory gap that puts people at risk, especially those on blood thinners, antidepressants, or immune suppressants.
Can I trust the "natural" label on supplements?
"Natural" has no legal meaning in supplement labeling. It doesn’t mean safer, purer, or more effective. Many supplements labeled "natural" contain synthetic ingredients or hidden pharmaceuticals. The FDA doesn’t define or enforce the term. Always check the ingredient list - not the marketing claims.
Are prenatal vitamins safer than other supplements?
Not necessarily. A 2021 study found that 40% of prenatal vitamins contain vitamin A levels above the recommended limit for pregnancy - and only 22% have clear warnings. Many contain retinol (the dangerous form) without specifying it. Always check the label for retinol or vitamin A in IU, and compare it to the 10,000 IU daily safety threshold.
Why don’t supplement labels show sodium content?
OTC medications must list sodium per serving because it affects blood pressure and heart health. Supplements don’t. This is a serious oversight for people with hypertension, kidney disease, or heart failure. Many multivitamins and energy supplements contain hidden sodium - sometimes over 100 mg per tablet - with no warning.
Is the FDA actively monitoring supplement safety?
The FDA only acts after harm is reported. They don’t test supplements before they’re sold. Between 2008 and 2020, the FDA found 776 supplements containing undeclared drugs - like steroids or erectile dysfunction meds - hidden in products sold as "natural." It took an average of 427 days to remove them. That’s reactive, not protective.

Annie Joyce
February 10, 2026 AT 23:38