When someone is diagnosed with cancer, they’re often faced with a flood of information - treatment options, side effects, survival rates. But one path that’s often overlooked, yet could change everything, is cancer clinical trials. These aren’t just experiments. They’re carefully structured research studies that have helped turn once-deadly cancers into manageable conditions. And if you’re considering your options, understanding how they work - and what you might gain - could be life-changing.
What Are the Phases of a Cancer Clinical Trial?
Cancer clinical trials don’t jump straight into giving new drugs to hundreds of people. They follow a strict, step-by-step system designed to protect patients while finding out what works. This system has five phases, each with a different goal, size, and risk level.
Phase 0 is the smallest and least common. It involves just 10 to 15 people. The goal isn’t to cure cancer, but to see if a drug even reaches cancer cells. Researchers give a tiny, non-therapeutic dose and track how the body absorbs it and how cancer responds. It’s like testing a key before cutting a new lock - you’re checking if it fits at all.
Phase I is where safety takes center stage. About 20 to 80 people join, usually those who’ve tried all other treatments. The main question: What’s the highest dose we can give without causing serious harm? Side effects are watched closely. Doses start low and creep up slowly. If a patient gets sick, the team backs off. This phase lasts a few months and is the riskiest because it’s often the first time humans are exposed to a new compound. But it’s also where many breakthroughs begin.
Phase II shifts focus to whether the treatment actually works. Around 50 to 100 people with a specific type of cancer join. Researchers look for signs like tumor shrinkage, slower growth, or longer survival. It’s not about beating every cancer - it’s about seeing if it helps this cancer, in this group. About half of drugs that enter Phase II don’t make it to the next stage because they don’t show enough benefit.
Phase III is the big test. Hundreds to thousands of people across multiple hospitals, sometimes in different countries, are randomly assigned to either the new treatment or the current standard. This is where you find out: Is this new thing better? Not just slightly better - meaningfully better? These trials can last 1 to 4 years. If the results are strong, the drug goes to the FDA for approval.
Phase IV happens after approval. Thousands more patients take the drug in real-world settings. Researchers watch for rare side effects that only show up over time, or how the drug works with other medications. This phase can run for decades. It’s how we learn that a drug that looked great in trials might cause heart problems in 1 in 1,000 people - information you’d never catch in a smaller study.
Why Would Someone Join a Clinical Trial?
People join for all kinds of reasons. Some are out of options. Others want to help future patients. And some just want the best possible care.
One major benefit is access. If standard treatments have stopped working, a clinical trial might be the only place to get a new drug. A woman in her late 50s with stage 4 melanoma, for example, joined a Phase II immunotherapy trial when chemotherapy failed. Three years later, she’s cancer-free. Her story isn’t rare. In a 2022 survey of trial participants, 78% said they got more frequent check-ups and closer monitoring than they did in regular care.
Another reason is quality. Patients in trials often get more attention. A research nurse checks in daily. Lab results are reviewed by a team. Side effects are tracked with precision. One participant said, “My oncologist spent 45 minutes explaining my bloodwork - something my regular doctor never did.”
And then there’s purpose. In a National Comprehensive Cancer Network study, 85% of participants said helping future patients gave them a sense of meaning. “Knowing my data might save someone else’s life made my chemo days feel less lonely,” shared a participant in a lung cancer trial.
What Are the Real Challenges?
It’s not all hope and progress. There are real hurdles.
First, eligibility. About 80% of cancer patients are turned away from trials. Why? Too many rules. Trials often require patients to have no other serious illnesses, normal liver and kidney function, or no prior treatments. That’s great for clean data - but it leaves out older people, those with other health issues, or people who’ve had prior therapies. The result? The people who benefit most from trials are often not the ones who need them most.
Logistics are another big barrier. One man in rural Wisconsin had to drive 3 hours each way for weekly infusions. He missed work, lost income, and couldn’t afford the gas. In the same ASCO survey, 37% of participants cited transportation as their biggest struggle. Time is another issue. Some trials require weekly visits for months. That’s impossible for people who work, care for kids, or live far from a cancer center.
Then there’s fear. Many people worry they’ll get a placebo - a sugar pill - instead of real treatment. In cancer trials, that almost never happens. Placebos are only used when there’s no standard treatment. More often, participants get either the new drug or the current best treatment. Still, 63% of potential participants say they’re anxious about randomization. That fear is real, and it stops people from even asking.
How Are Trials Changing Today?
The system isn’t stuck in the past. It’s evolving.
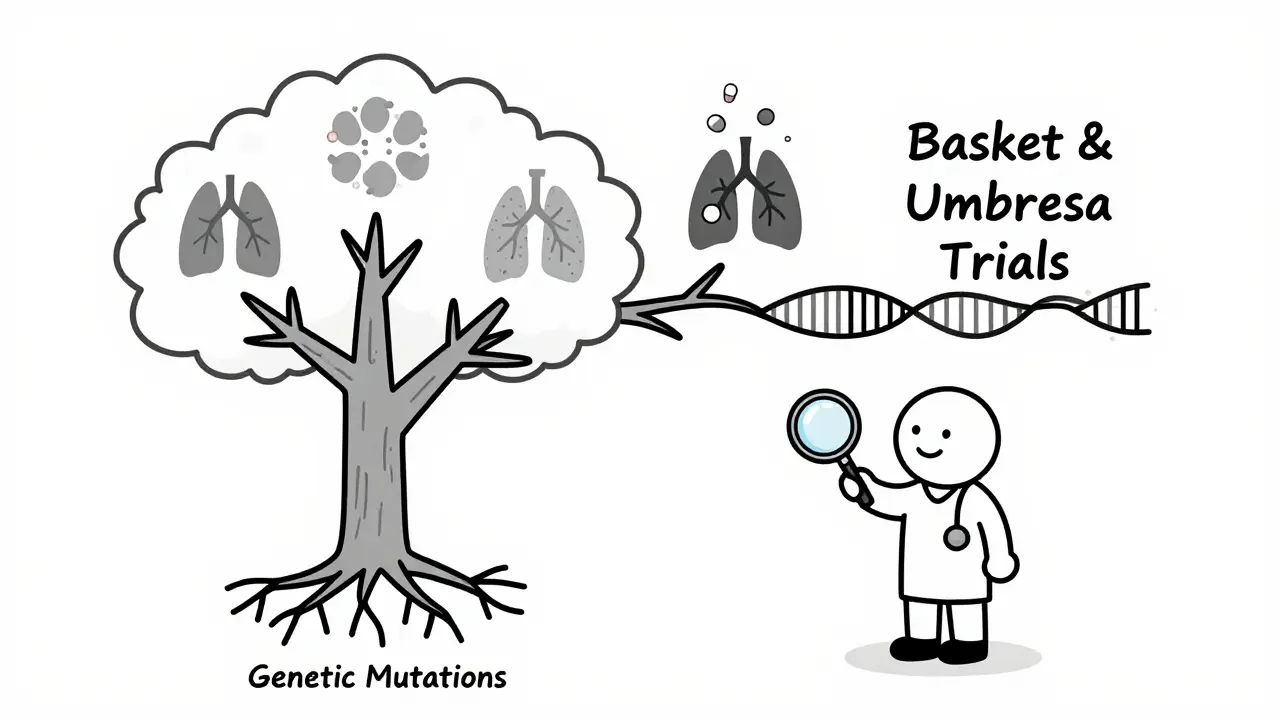
Master protocols - like basket and umbrella trials - are replacing old one-size-fits-all designs. Instead of testing one drug on one cancer type, a basket trial tests one drug on many cancers that share the same genetic mutation. An umbrella trial tests many drugs on one cancer type, matching each drug to a different mutation. The NCI’s MATCH trial, for example, matches patients to treatments based on their tumor’s DNA, not where the cancer started. This is precision medicine in action.
Remote monitoring is growing fast. In 68% of Phase III trials now, patients use wearables to track heart rate, sleep, and activity. They send blood test results from home. This cuts down on travel and makes trials more accessible.
And diversity is finally being addressed. Only 8% of trial participants are Black, even though they make up 13% of cancer cases. New initiatives are partnering with community clinics, offering transportation, and hiring staff who speak the same languages as patients. The goal: trials that reflect the real population.
Hybrid trials - mixing in-person visits with virtual check-ins - are planned by 45% of cancer centers by 2025. This could be a game-changer for rural patients.
What Should You Know Before Joining?
If you’re considering a trial, here’s what matters:
- Ask about randomization: Will you be randomly assigned? What are the two options?
- Ask about costs: Will your insurance cover routine care? Are travel or lodging expenses covered?
- Ask about time commitment: How many visits? How far do you have to travel?
- Ask about exit options: Can you leave anytime? What happens if you do?
Most cancer centers now have patient navigators - trained staff who help you understand your options, fill out paperwork, and even arrange rides. If your center doesn’t have one, ask for a referral. NCI-designated centers score 4.3 out of 5 on patient support; non-specialized centers score 3.1. The difference matters.
And remember: joining a trial doesn’t mean giving up on standard care. It means adding another option - one that’s being watched more closely than almost any other treatment.
Who Funds These Trials?
Clinical trials aren’t just run by drug companies. About 40% are funded by pharmaceutical firms. Another 35% come from academic research groups like the NCI. And 25% are government-funded. That means trials can be free or low-cost for patients. Many trials cover the cost of the experimental drug, scans, and even lab tests.
The global market for cancer trials is growing fast - from $28.7 billion in 2022 to an expected $52.3 billion by 2028. That growth means more trials, more options, and more hope.
Final Thoughts
Cancer clinical trials aren’t a last resort. They’re a pathway - sometimes the best one. They’ve brought us immunotherapy, targeted pills, and survival rates that were unimaginable 20 years ago. And they’re still changing. More inclusive. More flexible. More focused on real people, not just data points.
If you’re facing cancer, ask your doctor: “Are there trials for my type?” Don’t assume you’re not eligible. Don’t assume it’s too risky. Ask. Research. Talk to a navigator. You might be surprised by what’s out there - and how much it could mean for you, and for others after you.
Are clinical trials safe for cancer patients?
Yes, but with careful oversight. Every trial follows strict rules set by the FDA and international guidelines. Phase I trials start with tiny doses and increase slowly, watching for side effects. Independent safety boards review data regularly. If a treatment is too dangerous, the trial stops. While risks exist - especially in early phases - the system is designed to protect patients better than ever before.
Can I join a clinical trial if I’ve had previous cancer treatments?
Sometimes. Many trials exclude patients who’ve had prior treatments because it makes results harder to interpret. But not all. Some trials specifically recruit people who’ve tried standard therapies. Others are designed for second- or third-line treatment. Ask your oncologist or a trial navigator - eligibility isn’t always obvious.
Will I get a placebo instead of real treatment?
In cancer trials, placebos are rarely used alone. If a placebo is part of the study, you’ll still get the current standard treatment. For example, you might get chemotherapy plus either the new drug or a placebo. You won’t be left without treatment. The goal is to see if the new drug adds benefit - not to withhold care.
How long do cancer clinical trials last?
It varies by phase. Phase I lasts a few months. Phase II usually runs 6 to 12 months. Phase III can take 1 to 4 years. Phase IV continues for years after approval. For participants, your personal involvement depends on the trial design. Some require weekly visits for months; others may only need monthly check-ins. Always ask about the expected time commitment before joining.
Do I have to pay to join a clinical trial?
Usually not. The trial sponsor typically covers the cost of the experimental treatment, special tests, and extra monitoring. Routine care - like blood work or scans you’d get anyway - is often covered by insurance. Some trials also help with travel, lodging, or childcare. Always ask for a written cost breakdown before agreeing to join.

Jason Pascoe
February 13, 2026 AT 08:54