When you pick up a prescription at the pharmacy and see a different name on the bottle than what your doctor wrote, it’s natural to wonder: is this generic drug really the same? You’re not alone. Millions of people in the U.S. and around the world take generic medications every day, but doubts still linger. Some think generics work slower. Others worry they’re weaker. A few even believe they’re made in cheaper factories with lower standards. The truth? Bioequivalence testing is the science that answers all those questions-and it’s far more rigorous than most people realize.
What Bioequivalence Testing Actually Measures
Bioequivalence testing doesn’t just check if two pills look alike. It doesn’t even stop at checking if they contain the same active ingredient. It asks one critical question: Does the generic drug get into your bloodstream the same way, and in the same amount, as the brand-name version?
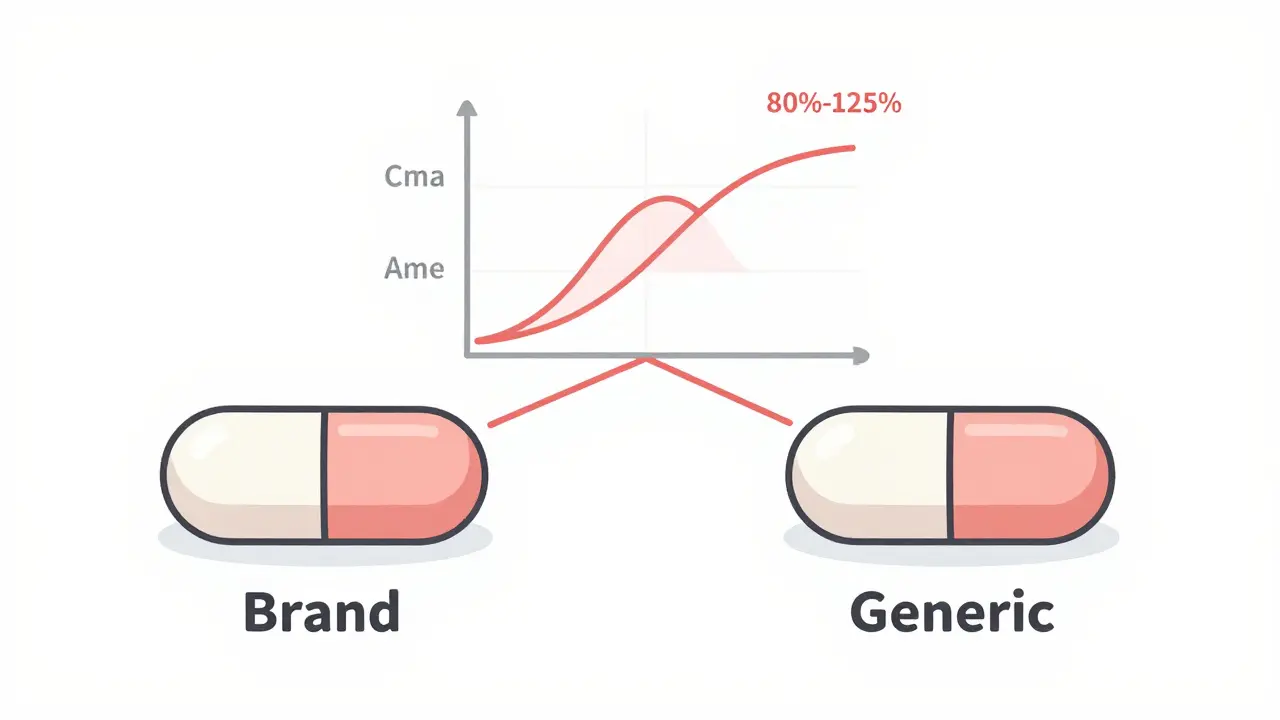
The answer comes from studying how your body handles the drug. Researchers give a group of 24 to 36 healthy adults both the brand-name drug and the generic version, usually in separate sessions spaced a week apart. Blood samples are taken over several hours to track how quickly the drug is absorbed and how long it stays in the system. Two key numbers are measured: Cmax (the highest concentration reached in the blood) and AUC (the total amount of drug absorbed over time).
The FDA requires that the generic’s Cmax and AUC fall within 80% to 125% of the brand-name drug’s values. That’s not a random range. It’s based on decades of clinical data showing that if two drugs stay within this window, their effects on the body are essentially identical. A 10% difference? That’s within normal variation between two doses of the same drug taken on different days. A 30% difference? That’s when you start seeing real changes in how the drug works.
Why This Matters More Than You Think
Most people think drug effectiveness is about strength-like a stronger battery. But drugs aren’t batteries. They’re chemicals that need to reach the right place at the right time. Take a blood pressure pill: if it’s absorbed too slowly, your pressure might spike before the drug kicks in. If it’s absorbed too fast, you might get dizzy. Bioequivalence testing ensures that doesn’t happen.
And it’s not just about absorption. The body breaks down drugs at predictable rates. If a generic drug clears from your system too quickly, you might need to take it more often. Too slowly, and you risk side effects building up. Bioequivalence testing makes sure the timing matches.
For drugs with a narrow therapeutic index-like warfarin, lithium, or levothyroxine-the margin for error is tiny. Even small differences can cause serious problems. That’s why these drugs often have tighter bioequivalence standards. The FDA doesn’t treat them the same as a common painkiller. They require more data, sometimes multiple studies, and extra scrutiny before approval.
How Generic Drugs Are Approved (Without Repeating Clinical Trials)
Brand-name drug companies spend over a billion dollars and 10 to 15 years testing their drugs in thousands of patients. Generic makers don’t do that. Instead, they use a shortcut called the Abbreviated New Drug Application (ANDA). But don’t let the word “abbreviated” fool you. It doesn’t mean less rigorous. It means they’re building on proven science.
The ANDA process starts with pharmaceutical equivalence: same active ingredient, same dose, same form (pill, liquid, injection). Then comes dissolution testing-checking if the generic breaks down in the lab the same way the brand does. If it passes, they move to bioequivalence studies with people. Only after all that does the FDA approve it.
The entire process takes 10 to 12 months on average. That’s faster than a new drug, but it’s still a full regulatory review. The FDA inspects the manufacturing facility, reviews every batch of data, and checks for consistency. In 2022, the agency inspected over 1,200 generic drug plants worldwide-more than half of them outside the U.S.

What Bioequivalence Testing Doesn’t Prove
Bioequivalence testing is powerful, but it’s not perfect. It doesn’t prove the generic works the same in every single person. It doesn’t test long-term side effects. And it doesn’t measure how the drug acts in specific tissues-like the lungs for inhalers or the skin for creams.
That’s why inhalers, nasal sprays, and topical creams are trickier. You can’t easily measure drug levels in the lungs or on the skin. For these, the FDA sometimes requires clinical endpoint studies-like measuring lung function after using an asthma inhaler-instead of blood tests. The EMA and other agencies have similar rules. These are complex products, and regulators know it.
Also, bioequivalence doesn’t mean identical. Generics can have different fillers, colors, or shapes. That’s why some people notice a change in how the pill tastes or how it feels in their throat. These inactive ingredients don’t affect how the drug works, but they can cause minor reactions in sensitive people. That’s not a failure of bioequivalence-it’s just a difference in formulation.
What Real People Experience
Surveys tell a clear story. In a 2022 Consumer Reports study of 1,200 people, 87% said they saw no difference between their generic and brand-name drugs. Nine percent said the generic worked better. Only 4% said it worked worse.
On Reddit’s r/pharmacy community, a thread with over 1,400 comments showed the same pattern: 78% reported no change. The few who did notice a difference mostly pointed to stomach upset or headaches-likely from new inactive ingredients, not the active drug.
Still, myths persist. A 2021 study found 32% of patients believed generics were less effective. Why? Because they remember when they switched and felt “off.” But correlation isn’t causation. Sometimes, switching drugs-even to an identical generic-triggers anxiety. Other times, it’s a placebo effect. Or it’s the brand-name drug they’ve been taking for years, and their body just got used to it.
The truth? If your generic works, it’s working. If you feel worse after switching, talk to your pharmacist. It might be the filler. It might be your body adjusting. But it’s rarely because the drug itself is weaker.
The Bigger Picture: Cost, Access, and Global Standards
Without bioequivalence testing, generic drugs wouldn’t exist. And without generics, healthcare would be unaffordable. In 2020, generics saved the U.S. healthcare system $313 billion. They make up 90% of all prescriptions but cost only 23% of what brand-name drugs do.
That’s why the global generic market is growing fast-projected to hit $781 billion by 2030. Countries like India and China produce most of the world’s generics, but they’re held to the same standards. The FDA, EMA, and other regulators share data and inspection protocols. The International Council for Harmonisation ensures that a generic approved in the U.S. meets the same bioequivalence rules as one approved in Europe or Japan.
And the science is evolving. The FDA is now using computer models to predict how drugs behave in the body-reducing the need for human studies in some cases. This isn’t cutting corners. It’s using better tools to get the same result: confidence that the drug works the same way.
Bottom Line: Trust the Science
Bioequivalence testing isn’t a loophole. It’s a carefully designed, evidence-based system that ensures generic drugs are safe, effective, and interchangeable with brand-name versions. The FDA, EMA, and other global regulators don’t approve generics lightly. They require proof-real, measurable proof-that the drug performs the same in your body.
If you’re concerned about your generic medication, ask your pharmacist. They can explain what’s in it and why it’s approved. If you notice a real change in how you feel, don’t assume the drug is weaker. Talk to your doctor. But don’t let fear stop you from saving money and getting the care you need.
The science is clear: bioequivalence works. For the vast majority of drugs, generics are not just cheaper-they’re just as good.
Are generic drugs really as strong as brand-name drugs?
Yes. Generic drugs contain the same active ingredient in the same strength as the brand-name version. Bioequivalence testing proves they deliver the same amount of drug into your bloodstream at the same rate. The FDA requires that the generic’s absorption falls within 80% to 125% of the brand’s-meaning the difference is clinically insignificant.
Why do some people say generics don’t work as well?
Most often, it’s not the drug itself. Some people react to inactive ingredients like dyes or fillers, which can cause mild side effects like stomach upset or headaches. Others feel worse after switching because they expect to. Anxiety can mimic side effects. In rare cases, a batch might have a manufacturing issue-but that’s why the FDA inspects factories regularly. If you notice a real change, talk to your pharmacist before stopping the medication.
Do generic drugs take longer to start working?
No. Bioequivalence testing measures how fast the drug enters your bloodstream (Cmax). If the generic met the 80%-125% range, it absorbs just as quickly as the brand. If you feel a delay, it might be psychological, or the pill might be coated differently for taste or stability-but not for slower absorption.
Are all generic drugs tested the same way?
No. Most pills and capsules use blood tests (pharmacokinetic studies). But for inhalers, creams, or eye drops, it’s harder to measure drug levels in the body. For these, regulators require clinical endpoint studies-like measuring lung function or skin improvement-instead of blood samples. Complex drugs like extended-release pills may need multiple bioequivalence studies under different conditions.
How does the FDA make sure generics are safe over time?
The FDA doesn’t approve a generic and walk away. They inspect manufacturing facilities annually-over 1,200 worldwide. They monitor adverse event reports from patients and doctors. If a pattern of problems emerges, they can pull the drug or require new testing. Generic manufacturers must report every batch and maintain strict quality controls. The system is designed to catch problems before they become widespread.
LOUIS YOUANES
January 28, 2026 AT 17:50